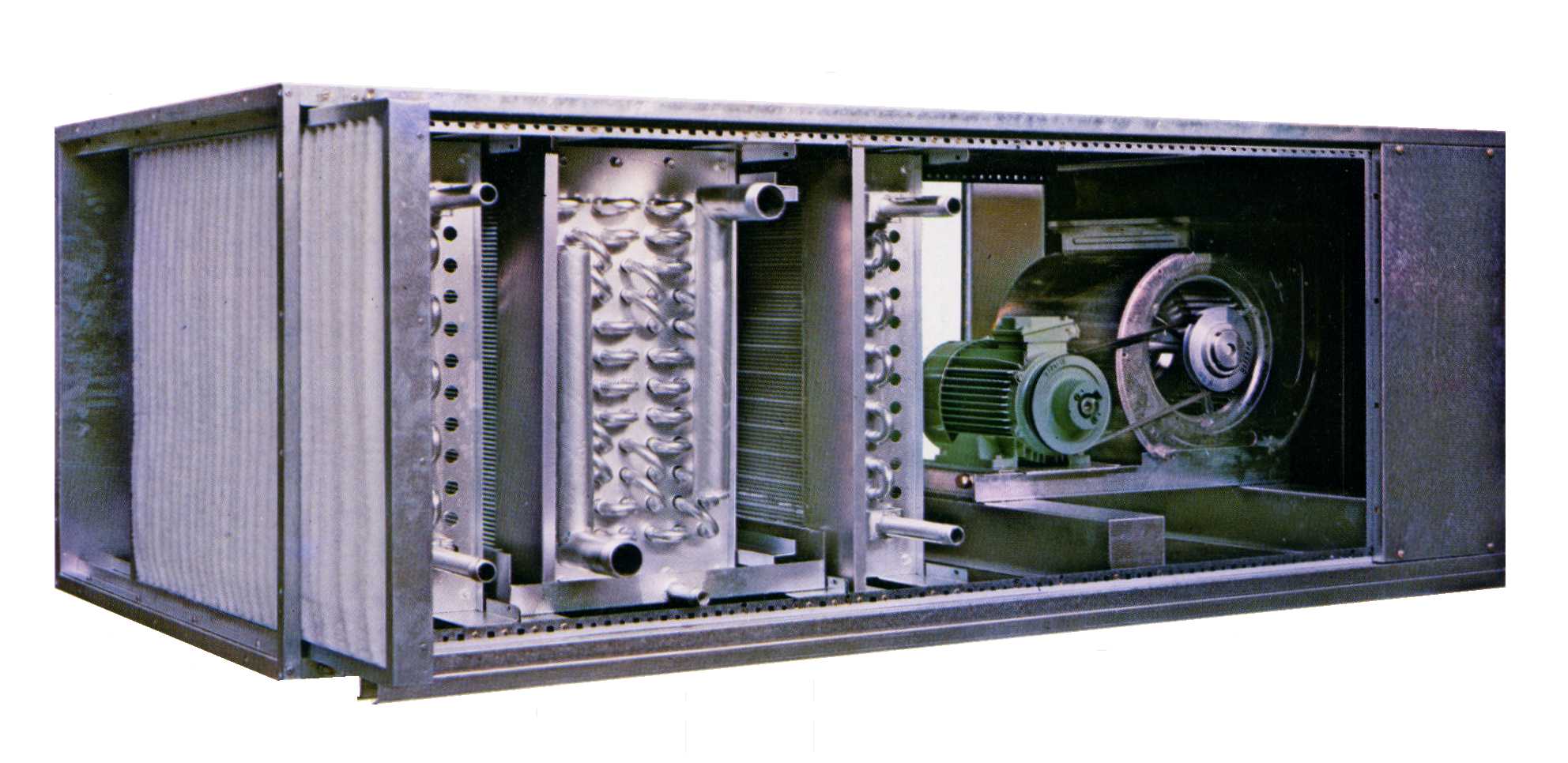
There are different sizes and types of Air Handling Units. Some will fit in a closet or within a ceiling space, while others will require a mechanical room or will need to be placed on the roof of a building. Within all of them, however, there is at least one fan and one method of conditioning the air.
These are the basic components in an AHU: Fans, Coils, Filters, Heaters, Humidifiers, Dampers, Mixers, and Enthalpy/Desiccant Wheels. There are other fixtures in an AHU for controls purposes, but basically these are the components you will see on most AHUs.